Microdigest 3
Round-up of CTAD
Here we summarize the key data releases from CTAD 2024 focusing on novel biomarkers and anti-amyloid therapies.
Screening for clinical trials
Key takeaways:
- p-tau217 ratio is an appropriate biomarker for reducing amyloid-β positron emission tomography (PET) screening failure rate across all racial and ethnic groups.
- Lower rates of plasma eligibility suggest differential prevalence of amyloid abnormality in racial and ethnic underrepresented groups contributing to their underrepresentation in anti-amyloid treatment trials.
- Tau PET is a good trial endpoint strongly correlating with cognition.
The AHEAD 3-45 study: design and results of a novel screening process for a preclinical AD trial (Abstract: LBS1)
The AHEAD 3-45 study comprises two sister trials:
- A3 is a phase 2, 4-year trial of intravenous lecanemab (5 mg/kg titration followed by 10 mg/kg monthly for 208 weeks) versus placebo in cognitively unimpaired individuals who have intermediate amyloid-β PET (20–40 centiloids [CL]). The aim is to prevent cognitive decline (Preclinical Alzheimer’s Cognitive Composite 5 [PACC-5]) over a 4-year period.
- A45 is a phase 3, 4-year trial of intravenous lecanemab (5 mg/kg biweekly for 6 weeks followed by 10 mg/kg biweekly for 86 weeks and then monthly for 120 weeks) versus placebo in cognitively unimpaired individuals who are amyloid-β PET positive (≥40 CL). The aim is to slow amyloid-β accumulation (amyloid PET and tau PET).
The screening period has just been completed.
Screening plasma biomarkers, amyloid and tau PET imaging in the AHEAD 3-45 study (Presentation 2)
Presenter: Reisa Sperling (USA)
- Screening process for both A3 and A45:
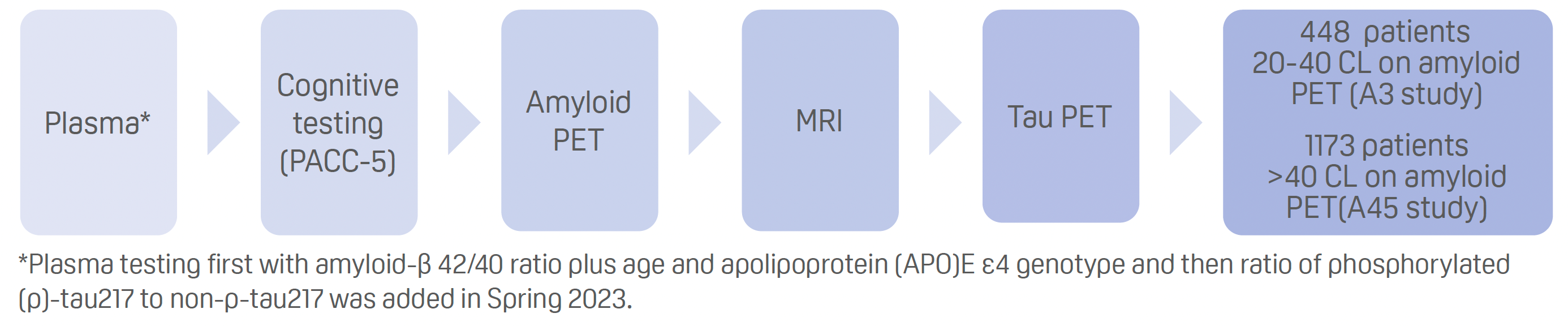
Results:
- Including plasma testing for the amyloid-β42/40 ratio reduced the amyloid PET failure rate for predicting more than 11 CL from around 75% to about 55%.
- The addition of the p-tau217 ratio further reduced the amyloid PET failure rate for predicting more than 18 CL to about 40–45%.
- Plasma p-tau217 ratio achieved an area under the receiver operative characteristic curve of 0.95 for amyloid PET over 20 CL eligibility.
- Plasma testing with the full plasma testing model reduced the amyloid PET failure rate for predicting more than 40 CL to 25%.
- Medial temporal lobe and neocortical tau PET were higher in A45 than A3 study participants.
- Tau PET showed the strongest correlation with cognition on PACC-5.
“Early rise in p-tau217 ratio may prove useful for even earlier interventional trials aimed at preventing future amyloid positivity,” said Sperling. “Findings support tau PET as a key endpoint, serving as a potential bridging outcome between imaging, biomarkers, and cognition across A3 and A45.”
Racial and ethnic differences in plasma p-tau217 biomarker eligibility rates in a preclinical AD Trial (Presentation 3)
Presenter: Doris Molina Henry
- Understanding the ability of the plasma biomarker algorithm, during the window when the p-tau 217 ratio was added, to detect amyloid PET eligibility at more than 18 CL in different racial and ethnic underrepresented groups.
Results:
- Lower plasma eligibility rates across all racial and ethnic underrepresented groups compared with non- Hispanic White adults.
- PET eligibility rates among plasma-eligible participants were comparable for the different racial/ethnic groups, with no significant differences.
| Racial/ethnic group | Number | Plasma eligibility rate | % of plasma eligible patients who were also PET eligible |
|---|---|---|---|
| Non-Hispanic White | 4832 | 27% | 71% |
| Hispanic White | 877 | 19% | 63% |
| Hispanic Black | 62 | 11% | 75% |
| Non-Hispanic Asian | 155 | 15% | 50% |
| Non-Hispanic Black | 511 | 19% | 68% |
“Lower rates of plasma eligibility suggest a differential prevalence of amyloid abnormality in these groups […] suggesting there are lower levels of amyloid in individuals from racially and ethnically underrepresented groups contributing to their underrepresentation in anti-amyloid trials,” said Henry.
“PET eligibility was the same across groups supporting that the same plasma prediction algorithms were appropriately applied across racial and ethnic groups,” she added.
“This suggests […] that other factors may explain higher dementia risk in individuals from racial and ethnic underrepresented groups who have lower amyloid prevalence.”
Biomarkers and AD diagnosis
Key takeaways:
- β-synuclein may represent a new synaptic blood-based biomarker in Alzheimer’s disease (AD).
- Time spent at each 0.5-unit of the Clinical Dementia Rating Scale–Sum of Boxes could help evaluate treatment response.
- A multianalyte blood biomarker test may aid diagnostic decision-making.
- Racial/ethnic disparities in amyloid positron emission tomography (PET) positivity and clinical stage at diagnosis need addressing to improve access to treatment.
- Strategies need to be developed to facilitate the interpretation of p-tau217 performance in the context of comorbid medical conditions such as chronic kidney disease.
- Different roles of blood-based biomarkers could pave the way for a stepwise diagnostic approach to AD.
- Further testing recommended when cerebrospinal fluid (CSF) and PET measures differ on amyloid-β positivity before recommending anti-amyloid therapy.
Early increase of the synaptic blood marker β-synuclein in asymptomatic individuals with autosomal dominant Alzheimer’s disease (Abstract ID OC14)
Presenter: Patrick Oeckl (Germany)
- Immunoprecipitation mass spectrometry was used to quantify the pre-synaptic protein β-synuclein in blood samples from 178 participants of the Dominantly Inherited Alzheimer Network (DIAN) study.
- Included 31 symptomatic mutation carriers, 78 asymptomatic mutation carriers, and 69 cognitively unimpaired mutation non-carriers.
Results:
- β-synuclein levels were significantly higher in asymptomatic patients with autosomal-dominant AD than cognitively unimpaired individuals and highest in symptomatic patients.
- Blood β-synuclein levels started to rise in the mutation carriers approximately 11 years before symptom onset.
- Ranking according to when fluid biomarker levels significantly diverge between mutation carriers and non-carriers, from earliest to latest:
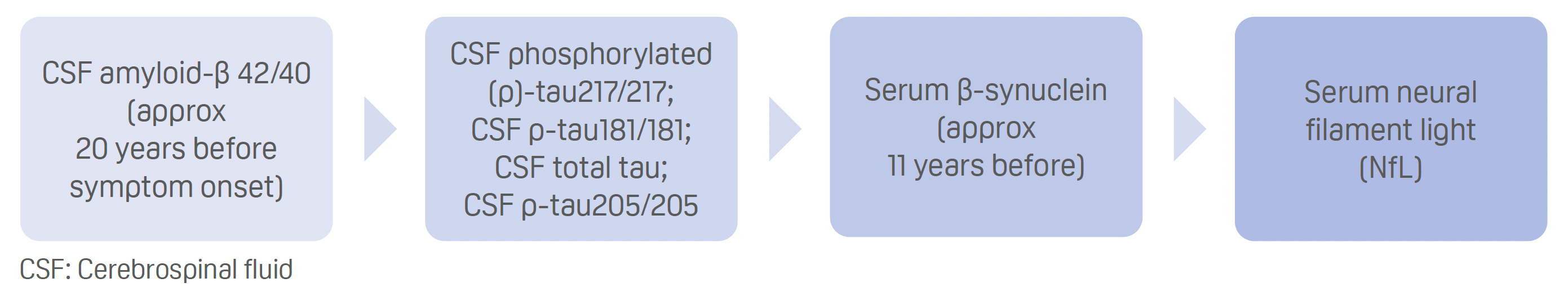
- Serum β-synuclein trajectory precedes imaging measures of cognitive impairment, brain atrophy, and hypometabolism.
- Serum β-synuclein had moderate ability to predict amyloid-β positivity (area under the receiver operating characteristic curve of 0.75).
- Serum β-synuclein significantly correlated with cognitive impairment.
Blood β-synuclein could be an “easily accessible synaptic marker for diagnosis, prognosis, [and] drug development,” said Oeckl.
Clinical progression on CDR-SB: residence time at each level in the DIAN and ADNI cohorts (Abstract OC19)
Presenter: Guoqiao Wang (USA)
- The length of time people spend at each 0.5-unit Clinical Dementia Rating Scale–Sum of Boxes (CDR- SB) before they progress to the next level, termed residence time, was examined in two cohorts – DIAN (autosomal-dominant AD) and ADNI (sporadic AD) using random effects model for disease progression.
Results:
- Autosomal-dominant AD: Residence time per 0.5 unit CDR-SB of 0.5 years or less when CDR-SB reaches 3 points or higher.
- Sporadic AD: Residence time per 0.5 unit CDR-SB of 0.5 years or less when CDR-SB reaches 3.5 points or higher.
- Residence time to go from CDR-SB score of 0.5 to 3.5 points: average 6.5 years for autosomal- dominant AD and 5.6 years for sporadic AD, a difference of approximately 0.9 years.
Using residence time, a treatment effect could be calculated for patients in the open label extension phase of the Clarity AD trial of lecanemab, for example:
- From a baseline CDR-SB score of 3.17 points, it was estimated that placebo-treated patients would take 2.3 years to decline by 3.09 points and those treated with lecanemab would take 3.0 years, a delay in disease progression of 0.7 years.
Residence time at each 0.5-unit level in CDR-SB “could provide a standardized, alternative way to interpret and evaluate the treatment effect,” said Wang.
Discrepancies between CSF and PET determinations of elevated brain amyloid and their prognostic significance (Abstract: LB10)
Presenter: David Knopman (USA)
- Cognitive decline following discordant CSF and PET measures of amyloid-β positivity was assessed in 587 participants of the ADNI cohort who had mild cognitive impairment.
Results:
- Discordant test results were uncommon occurring at a rate of approx. 5%.
- Patients who tested negative for both CSF and PET amyloid and those with discordant test results (tested negative on either) had similar cognition at baseline, and cognition was better compared with those testing positive on both measures.
- For patients testing positive for CSF amyloid but not PET amyloid, CSF p-tau/amyloid-β ratios were substantially lower than those for patients testing positive on both measures.
- Over a median 4 years of follow-up, patients with discordant test results did not have a significant decline in scores on the Rey auditory Verbal Learning Test Sum of Trials 1–6 or Clinical Dementia Rating Scale–Sum of Boxes, unlike patients testing positive on both measures.
- Rates of incident dementia over follow-up were only significantly elevated in patients who tested positive on both measures (17.3 per 100 person–years), while patients testing negative or with discordant results on both measures had similar rates (ranging from 1.9 to 3.0 per 100 person–years).
“Because persons with MCI CSF positive/PET negative pattern did not exhibit group-wise decline, additional evidence for the likelihood of disease progression should be obtained before recommending them for anti-amyloid monoclonal antibody treatment,” said Knopman.
An evaluation of the impact of a multianalyte blood biomarker test for evaluating cognitive impairment: results of the QUIP II clinical utility study (Abstract OC32)
Presenter: Joel Braunstein (USA)
- Twelve clinicians treating 203 cognitively impaired patients were surveyed to assess the use of PrecivityAD2 (C2N Diagnostics, St Louis, Missouri, USA) in clinical management and decision making.
- The blood test quantifies plasma levels of amyloid-β42 and 40 as well as p-tau217 and non-p-tau217 to give an Amyloid Probability Score (APS)2, from 0–100 points, where a score of 0–47 signifies a negative result and a low likelihood of amyloid positivity and a score of 48–100 points a positive result and a high likelihood.
Results:
- 51% of patients tested negative.
- 99% concordance with intended use.
- 75% change in AD diagnostic certainty, drug therapy, or further brain amyloid evaluations.
- Weak correlation between APS2 result and clinician-reported probability of AD before using the blood test, and a strong correlation afterwards.
- For negative patients, clinician-reported AD probability decreased from 53% before using the blood test to 11%, and for positive patients, increased from 65% to 93%.
- There was a significant decrease in AD drug prescribing among negative patients before vs after using the blood test and a significant increase among positive patients.
- Additional brain amyloid testing decreased by 70% in negative patients and 26% in positive patients.
“We believe […] the PrecivityAD2™ blood test led to clinically meaningful changes in decision-making around AD diagnostic certainty, drug therapy management, and additional amyloid evaluation among patients evaluated for cognitive impairment,” said Braunstein.
Presenter: Gil Rabinovici (USA)
Differences in amyloid PET results and social determinants of health by race/ethnicity: results from New IDEAS (Abstract OC08)
- Rates of amyloid PET positivity, clinical stage, and social determinants of health were compared across 4845 New IDEAS participants with mild cognitive impairment (MCI; 64%) or dementia (37%), of whom 21% self-identified as Black/African American (BAA), 18% as Latino/Hispanic (LAT), and 61% as another race or ethnicity (NBL).
Results
The BAA and LA groups were:
- a significant 28% less likely than the NBL group to be amyloid-PET positive, with respective rates of 60.7% and 60.8% versus 68.2%;
- more likely to have vascular comorbidities, such as hypertension (73.3 and 61.5 vs 54.3%) and diabetes (29.9% and 29.8% vs 16.3%);
- more likely to present with dementia, relative to MCI, than the NBL group, at a rate of 47.4% and 43.5% versus 30.5%;
- more likely to have an atypical presentation and therefore harder to diagnose (36.0% and 35.55 vs 26.6%);
- more likely to have lower mean mini–mental state examination scores on average at baseline than the NBL group (21 and 21 vs 25 points).
“Addressing these modifiable disparities is critical to enhancing equity in access to care and implementation of dementia prevention strategies,” said Rabinovici.
Performance of plasma p-tau217 in an African American cohort: findings from the African Americans fighting Alzheimer’s in midlife study (Abstract: OC13)
Presenter: Gilda Ennis (USA)
- The accuracy of plasma p-tau217 to detect amyloid PET positivity was assessed in 233 cognitively unimpaired adults identifying as non-Hispanic Black or African American from the AA-FAIM cohort who had at least one of the following comorbid conditions: impaired kidney function, cardiovascular disease, hypertension, diabetes, or obesity.
Results
- Higher plasma p-tau217 levels were significantly associated with mild (estimated glomerular filtration rate [eGFR] below 90 mL/min per 1.73 m2) and moderate-to-severe (eGFR below 60 mL/min per 1.73 m2) kidney function impairment, as well as cardiovascular disease (myocardial infarction, stroke, or congestive heart failure).
- Neither association could be explained by amyloid burden in small sub-samples of 38 and 64 individuals, respectively.
- Plasma p-tau217 levels were not significantly increased in patients who had stage 1 or 2 hypertension, diabetes, or obesity, after controlling for age and gender.
- The accuracy of plasma p-tau217 to detect amyloid PET positivity in 65 patients from the AA-FAIM cohort was 90% at a cutoff point of 0.35 pg/mL, with a sensitivity of 94% and a specificity of 80% (Global CEO initiative on AD recommends 90% and 85%, respectively, for primary care use of blood-based biomarkers).
“We need to investigate strategies to facilitate the accurate interpretation of plasma p-tau 217 in the context of medical conditions falsely increasing, or decreasing, p-tau217,” said Ennis.
Differential roles of Alzheimer’s disease plasma biomarkers in stepwise biomarker guided diagnostics: head-to-head comparison among an Asian population (Abstract: OC38)
Presenter: Daeun Shin (Republic of Korea)
- The differential roles of plasma markers were studied in 2984 Asian people (22% cognitively unimpaired, 68% AD cognitively impaired, 6% subcortical vascular cognitively impaired, 3% frontotemporal dementia) to see if they could be used as part of a stepwise strategy for more efficient biomarker-guided diagnosis of AD.
- Plasma biomarkers studied: amyloid-β 42/40; p-tau181; p-tau217; p-tau231; GFAP; NfL.
Results
| Best biomarker | Role | AUC |
|---|---|---|
| NfL | Distinguished patients with any cognitive impairment from unimpaired individuals | 0.71–0.94 |
| p-tau217 | Discriminated amyloid-β PET positivity in all groups | 0.88–0.95 |
| p-tau217 | Discriminated tau PET positivity in AD cognitively impaired patients | 0.90–0.91 |
| p-tau217 | Distinguished amyloid-β PET-positive patients with AD cognitive impairment from amyloid-β PET-negative patients with non-AD dementias. | 0.94–0.95 |
| p-tau217 and then GFAP | Predicted cognitive decline in cognitively unimpaired patients | N/A |
AUC: area under the receiver operating characteristic curve; N/A; not applicable; NfL; neurofilament light; p: phosphorylated; PET: positron emission tomography; GFAP: glial fibrillary acidic protein
“These findings underscore the importance of the differential roles of Alzheimer’s disease plasma biomarkers in a stepwise diagnostic approach,” said Shin.
Anti-amyloid therapies
Key takeaways:
- Appropriate use recommendations have been issued for donanemab to help identify appropriate candidates for treatment.
- Eligibility for anti-amyloid therapy is relatively low and could potentially be improved with broader screening including blood-based biomarkers.
- Real-world patients are being appropriately selected and receiving lecanemab in line with FDA approved labeling information.
- Lecanemab is well-tolerated by patients in the clinic and adherence rates are good.
Donanemab: appropriate use recommendations (Abstract: LB01)
Presenter: Gil Rabinovici (USA)
- The recommendations were devised by the AD and Related Disorders Therapeutic Workgroup based on donanemab clinical trial data, FDA prescribing information, and other relevant literature together with expert opinion.
Results
Patients eligible for donanemab treatment should have:
- Mild cognitive impairment (MCI) or mild dementia due to AD: Clinical stages 3–4 on the Global Deterioration Scale and a Mini–Mental State Examination (MMSE) score of 20–30 points.
- Evidence of amyloid-β pathology confirmed by either cerebrospinal fluid (CSF) biomarkers (amyloid-β 42/40 or phosphorylated (p)-tau 181/amyloid-β 42 ratios) or positron emission tomography (PET).
- Apolipoprotein (apo)E genotyping and magnetic resonance imaging (MRI) conducted prior to treatment.
Not eligible for donanemab treatment are:
- Patients with MRI evidence of severe white matter disease or significant cerebral amyloid angiopathy, such as more than four microbleeds, a macrohemorrhage more than 1 cm, or superficial siderosis.
- Patients taking anticoagulants.
Further recommendations:
- Thrombolytics should not be used during donanemab treatment; mechanical thrombectomy is feasible.
- Discontinuation of donanemab if MRI shows any macrohemorrhage, more than one area of superficial siderosis, more than 10 microhemorrhages, more than two episodes of amyloid-related imaging abnormalities (ARIA), or severe ARIA.
- Consider discontinuation of treatment at 12–18 months if follow-up amyloid PET is negative.
Rabinovici said that these are “recommendations, they are not guidelines or criteria, and as always when treating an individual patient, clinical judgment is paramount.”
Eligibility for anti-amyloid treatment in real world memory clinic populations (Abstract: OC22)
Presenter: Anna Matton (Sweden)
- The appropriate use recommendations for lecanemab were applied to a representative, real-world sample of 2126 patients from seven memory clinics in Sweden to see who would be eligible for anti- amyloid therapy.
Results:
- Mean age 77.1 years; 50.4% women.
- Mean MMSE was 27 points.
- 32% of patients had pathologic Fazekas scores.
- 62% had MCI and 24% had AD dementia.
- 17% of patients were taking anticoagulants and 17% were taking antiplatelets.
- Of 918 patients with available measures of CSF biomarkers, 34.3% had abnormal amyloid-β 42, 32.5% had abnormal phosphorylated (p)-tau, 50.4% had abnormal total tau, and 20.6% had abnormal neurofilament light.
- The ATN profiles for 774 of the patients were:
| A-T-N- | A–T–N+ | A+T–N+ | A–T+N– | A+T–N– | A–T+N+ | A+T+N– | A+T+N+ |
|---|---|---|---|---|---|---|---|
| 26.1% | 18.5% | 11.8% | 10.7% | 9.6% | 9.3% | 7.0% | 7.1% |
A: CSF Amyloid-β 42; T: CSF p-tau181, N: medial temporal lobe atrophy Highlighted profiles are those eligible for anti-amyloid treatment.
- Based on recommended inclusion and exclusion criteria, 86 individuals were eligible for anti-amyloid therapy – 9.4% of CSF eligible patients and 4.1% of the total patients.
- These patients comprised 10% of the MCI patients (total 64%) and 25% of those with AD dementia (total 36%).
“Overall, [a] relatively low proportion of patients would be potentially eligible,” said Matton. “Broader screening approaches including AD blood biomarkers could potentially increase the numbers.”
One-year experience on the use of lecanemab in clinical practice (Abstract: LBS2) Lecanemab treatment in real world settings in the United States (Presentation 1)
Presenter: Marwan Sabbagh
- Data assessed from the Komodo research database for 3155 patients who started lecanemab treatment (predominantly since October 2023) and had a mean of 129 days of follow-up and information on clinical activity 12 months prior to treatment.
Results
- Patients had a mean age of 75 years, 84.3% were White, 93.3% were from urban areas, 55.8% women.
- Majority had comorbid conditions, primarily dyslipidemia (54.4%) and hypertension (45.7%).
- 60.8% had MCI and 83.8% had AD.
- 67.6% were taking oral anti-AD medications (acetylcholinesterase inhibitors or memantine), 3.7% were taking anticoagulants, and 4.1% were taking antiplatelets.
- Average time from diagnosis to starting treatment was 4.9 months.
- Patients averaged two monthly infusions within 16.5 days of each other and the first MRI scan was conducted a mean of 46.7 days after treatment initiation.
- The rate of adherence – no more than 90 days between two infusions – was 85.1%.
Patients were receiving lecanemab “on label and on time,” said Sabbagh. They are “highly motivated to stay on lecanemab once they understand the consequences of treatment and nontreatment.”
Lecanemab use in clinical practice at an academic medical center (Presentation 2)
Presenter: Lawrence Honig
- Experience of treating 162 patients with lecanemab at the academic Irvine Medical Center in New York, USA.
Results
- Patients had a mean age of 73 years; 90% were White; 56% were women.
- Comorbid conditions were common, including strokes and bleeds in 2% and vascular malformations in 3%.
- Evidence of MCI or AD dementia confirmed by CSF biomarkers in 86%, by PET in 26%, and by both in 12%.
- 2% of patients had pacemakers and 1% were taking anticoagulants.
- Mean MMSE score was 23.6 points, ranging from 11 to 30 points.
- ApoE genotyping was carried out in 89%, while the remaining 11% declined.
- Risk discussions were carried out with all patients, only those with more than four microhemorrhages on pre-treatment MRI were excluded.
- An average of 13.1 infusions were received over an 18-month period and up to four post-treatment MRI scans.
- Nine unscheduled MRI scans were carried out because of suspected ARIA, one of which was positive.
- The rates of ARIA edema/effusion (ARIA-E) and ARIA microhemorrhage (ARIA-H) were 11% and 5%, respectively.
- ARIA-E typically occurred early, was mostly asymptomatic, and resolved in a few months with lecanemab dosing resumed.
- Treatment was paused in 9% of patients, predominantly due to ARIA-E.
- 16 of 17 cases of ARIA-E were asymptomatic.
- 8% of patients discontinued treatment: one with ARIA-E, two with ARIA-H, and one with both ARIA-E and ARIA-H who subsequently died, as well as eight due to burden, disinterest, insurance issues, or lack of benefit, and four who were lost to follow-up due to relocating.
The clinical experience with lecanemab “was not dissimilar to that in clinical trials,” said Honig. It was “safe and manageable” with “wide patient acceptance and compliance.”
ARIA
Key takeaways:
- Dose titration of donanemab could help reduce ARIA-E rates.
- ‘Off-target’ binding of anti-amyloid therapies to cerebral amyloid angiopathy rather than amyloid plaque may not explain differences in ARIA-E rates.
- AI could help make adverse event coding more efficient for clinicians.
- Variability for ultrafast MRI measures no greater than with standard scans.
The effect of different donanemab dosing regimens on ARIA-E and amyloid lowering in adults with early symptomatic Alzheimer’s disease: primary outcome results from TRAILBLAZER-ALZ 6 (Abstract: OC01)
Presenter: John Sims (USA)
- The phase 3b study looked at whether modifying the dosing regimen of donanemab could reduce ARIA-E rates in 843 patients with AD.
–
–
Standard titration – intravenous donanemab every 4 weeks at a dose of 700 mg for the first three infusions and then at 1400 mg for the fourth.
Modified titration regimen – intravenous donanemab 350 mg for the first infusion, 700 mg for the second infusion, 1050 mg for the third infusion, and 1400 mg for the fourth infusion.
Results
- By week 24, patients in the modified titration arm had a significantly lower rate of ARIA-E than those in the standard titration arm, at 13.7% versus 23.7%.
- There was a 94% probability of achieving at least a 20% reduction in ARIA-E rates with the modified titration, which met the primary study objective of more than 80%.
- On MRI at 24 weeks 86% of patients in the modified titration group were free of ARIA-E, compared with 76% of those in the standard titration group.
- There was no difference between the two titration regimens in terms of other serious adverse events or treatment discontinuations or treatment-related adverse events.
- There was one death in a patient with ongoing ARIA-E in the modified titration group due to cerebral intraparenchymal hemorrhage
- Amyloid-lowering was comparable with the modified and standard titration regimens (mean decreases from baseline of 56.3 and 58.8 centiloids, respectively).
The results suggest that “an enhanced titration approach may limit ARIA risk while maintaining sufficient amyloid reduction.”
Anti-amyloid antibody preference for vascular Aβ aggregates does not explain ARIA rates (Abstract: OC35)
Presenter: Andrew Stern
- The affinity with which aducanumab, donanemab, and lecanemab bind to “off-target” cerebral amyloid angiopathy relative to “on-target” plaque amyloid as a possible explanation for differing amyloid-related imaging abnormality with edema and/or effusion (ARIA-E) rates among the therapies was tested.
Results
- Previously reported ARIA-E rates:
- 35.2% for aducanumab (*2.8 fold difference)
- 24.0% for donanemab
- 12.6% for lecanemab (*2.8 fold difference)

- The preferential binding of antibodies synthesized from amino acid sequences to meningeal amyloid-β 40, which is CAA enriched, and parenchymal amyloid-β42, which is plaque enriched was increased by a mean:
- 1.51-fold with aducanumab
- 1.63-fold with donanemab
- 1.85-fold with lecanemab
- There was no significant difference between the three therapies, and the 0.83- to 1.8-fold preferential binding with aducanumab does not explain the 2.8-fold difference in ARIA-E rate compared with lecanemab.
Stern concluded that “antibody preference for meningeal amyloid-β 40-rich aggregates over parenchymal Amyloid-β 42-rich aggregates cannot explain differences in ARIA rates.”
Artificial intelligence-enabled safety monitoring in Alzheimer’s disease clinical trials (Abstract: OC36)
Presenter: Gustavo Jimenez-Maggiora (USA)
- The use of natural language processing and artificial intelligence (AI) to augment the clinician-based coding of 980 adverse events using data collected from eight completed trials of symptomatic AD patients was assessed.
Results
- AI outperformed traditional clinician-based coding overall (accuracy 88 vs 71%) and across the code frequency range, and improved accuracy at the reporting level (after expert clinical review and biostatistical analysis) by 3–13%.
- AI coding was comparable in a separate cohort (A4 Study) of pre-AD patients to that of clinician-based coding, at around 70%, but increased the accuracy at the reporting level by 3–5%.
- AI-based AE coding was more cost effective, with an approx. 80% cost reduction.
“Artificial intelligence-based coding can be performed instantaneously without loss of accuracy relative to clinician coding, reducing costs, and improving the availability of safety data,” said Jimenez-Maggiora.
An ultra-fast MRI protocol to aid diagnosis and treatment of Alzheimer’s disease (Abstract: OC34)
Presenter: Miguel Rosa-Grilo (UK)
- The variability of three neurologists interpreting a fast-imaging scan using wave-controlled advanced parallel imaging was compared with a standard clinical magnetic resonance imaging (MRI) scan (fast vs standard scans) in 90 individuals: 41.1% had no neurodegenerative brain disease; 21.1% had AD; 12.2% had frontotemporal lobar degeneration (FTLD); 8.9% had vascular cognitive impairment (VCI); and 16.7% had other diagnoses.
Results
- Fast scan reduces acquisition time by about 63% relative to a standard scan (6 min 29 sec vs 17 min 39 sec)
- The variability introduced by the fast scan was less than for the standard scan, providing evidence for noninferiority between the two methods.
Inter-rater reliability kappa coefficients* were:
- Normal: Fast scan=0.61; standard scan=0.65; between the scans: 0.80
- AD: Fast scan=0.38; standard scan=0.42; between the scans=0.72
- FTLD: Fast scan=0.79; standard scan=0.71; between the scans=0.95
- VCI: Fast scan=0.75; standard scan=0.75; between the scans=0.95
- Other diagnoses: Fast scan=0.48; standard scan=0.50; between the scans=0.76
* 0.21–0.40=fair; 0.41–0.60=moderate; 0.61–0.80=substantial
“Further studies are required to assess actual time savings in practice and ultra-fast implementations across different field strengths,” said Rosa-Grilo.
Click below to download the Microdigest 3 pdf from CTAD:
Download Microdigest 3 PDFYou can also download the Microdigest 2 pdf from AAIC:
Download Microdigest 2 PDFYou can also download the Microdigest from AD/PD & CONy:
Download Microdigest 1 PDF





