Microdigest 2
Round-up of AAIC
Here we summarize the key data releases from AAIC 2024 focusing on novel biomarkers and anti-amyloid therapies.
Plasma Biomarkers
Key takeaways:
Potential clinical applications:
- Clinical screening and selecting patients for treatment.
- Disease staging.
- Monitoring treatment response.
Considerations:
- The potential impact of comorbidities such as chronic kidney disease.
- The influence of racial differences on levels and cutoff points using assays.
- The need to standardize clinically relevant assays.
- When to test.
Evaluation of the prospective use of blood biomarkers for Alzheimer’s disease in primary and secondary care (Abstract ID: 88404)
Presenter: Oskar Hansson (Sweden)
Plasma biomarkers tested:
- Amyloid probability score (APS)2: plasma amyloid-β 42 to 40 ratio + plasma phosphorylated (p)-tau217 to non-p-tau217 ratio.
- Plasma p-tau217 to non-p-tau217 ratio alone.
Results:
- Plasma p-tau217 ratio alone had comparable accuracy to APS2.
- Diagnostic accuracy of both plasma biomarkers around 89% to 92% across primary and secondary care settings, in single batch and prospective analyses, when using single and dual threshold cutoffs, and for cerebrospinal fluid (CSF) amyloid pathology as well as clinical AD.
- Respective positive and negative predictive values (PPVs and NPVS) ranged from 86% to 92% and 90% to 97%.
- Blood biomarkers significantly improved on the standard-of-care diagnostic workup made by primary care physicians and dementia specialists.
- Low rates of “intermediate” patients based on the dual threshold of 95% specificity (high risk) and 95% sensitivity (low risk): primary care 4–13% and secondary care 6–11%.
Utility of Plasma Biomarkers in Screening for Brain Amyloid in the 1Florida Alzheimer Disease Research Center (ADRC) (Abstract ID: 91578)
Presenter: Ranjan Duara (USA)
- Ethnicity/race of participants: 56% Hispanic and 96% White; 3% Black; 1% other.
- Blood-based biomarkers tested: amyloid-β 42/40 ratio; p-tau217; glial fibrillary acidic protein (GFAP); and neurofilament light (NfL).
- A combination of biomarkers, APOE genotype, and hippocampal atrophy predicted positive amyloid positron emission tomography (PET) with “high accuracy,” in logistic regression models, using the Youden’s index sensitivity and specificity cutoff and accounting for age and sex.
- p-tau217 performed the best as a standalone plasma biomarker.
| AUC | Sensitivity | Specificity | |
|---|---|---|---|
| APOE ε4+, hippocampal atrophy+ (base) | 0.78 | 71% | 76% |
| Base plus all plasma biomarkers | 0.96 | 93% | 87% |
| Base plus amyloid-β 42/40 ratio | 0.88 | 80% | 82% |
| Base plus p-tau217 | 0.94 | 92% | 84% |
| p-tau217 | 0.92 | 85% | 89% |
| Amyloid-β 42/40 ratio | 0.82 | 77% | 81% |
| Amyloid-β 42/40 ratio plus p-tau217 | 0.94 | 93% | 85% |
Abbreviations: AUC, area under the receiver operating characteristic curve; APOE, apolipoprotein E.
Use of plasma p-tau217 as a pre-screening method for detecting amyloid-PET positivity in cognitively unimpaired participants: A multicenter study (Abstract ID: 85773)
Presenter: Gemma Salvadό (Sweden)
- Cut-offs: specificity 90.0%, 95.0%, 97.5%.
- Results after adjusting for age and APOE status:
| p-tau217 only | Subsequent CSF amyloid-β 42/40 in patients positive for p-tau217 | |
|---|---|---|
| Positive predictive value | 72.9–81.2% | 90.8–95.3% ↑ |
| Negative predictive value | 82.5–86.2% | 82.8–86.7% |
| Accuracy | 82.4–83.8% | 84.0–87.3% ↑ |
| Overall rate of amyloid positivity | 10.9–18.1% | 9.3–14.3% ↓ |
| Probability of being assessed as positive on both p-tau217 and CSF | - | 79.4–85.2% |
- “Plasma p-tau217 could be used, either as stand-alone biomarker, or as an initial step before CSF biomarkers (reducing their need by ~80–90%), for pre-screening in clinical trials of preclinical AD depending on the certainty needed for Aβ-PET positivity,” said Salvadό.
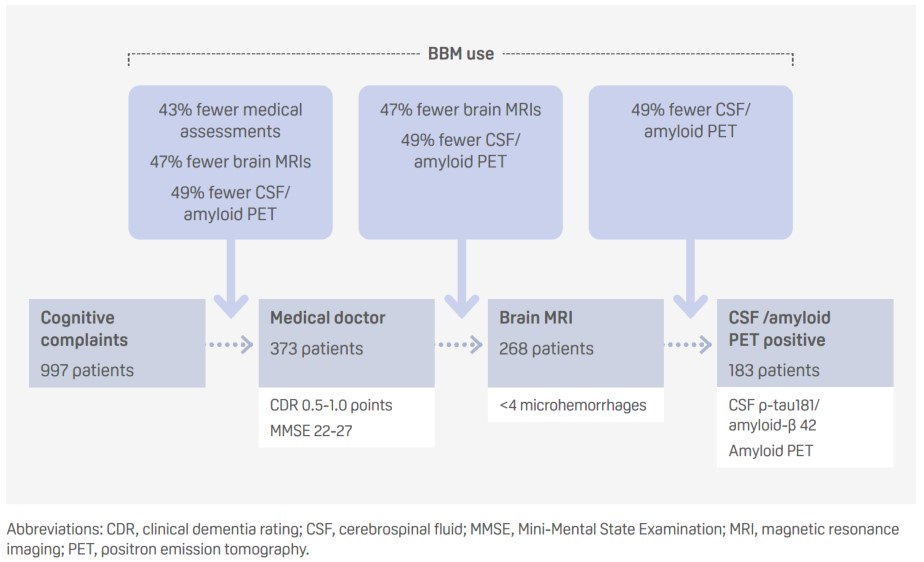
Using a blood-based biomarker panel for Alzheimer’s disease to determine eligibility for disease modifying treatment in a memory clinic setting: three scenarios (Abstract ID: 91700)
Presenter: Sinthujah Vigneswaran (the Netherlands)
- The use of blood-based biomarkers (BBMs) at different stages of the standard diagnostic trajectory could increase the efficiency of the process negating the need for additional testing and thereby reducing costs.
- p-tau217 detected amyloid positivity, based on a Youden’s index cutoff of 92% specificity, with a sensitivity of 86%, a specificity of 87%, and with false positive and negative rates of 7% and 32%, respectively.
Evaluating the interchangeability of blood-based biomarkers and amyloid-PET for identifying patients with Alzheimer’s pathology (Abstract ID: 91465)
Presenter: Samantha Burnham (USA)
Plasma biomarkers tested:
- PrecivityAD®, which combines amyloid-β42/40 levels, APOE ε4 and age.
- p-tau217.
Plasma stratification:
- Amyloid-β42/40: absence of amyloid plaques, presence of amyloid plaques, or intermediate.
- p-tau217: negative, positive, or indeterminate.
Results after ruling out 14.0% of intermediate patients on PrecivityAD® and 18.6% on p-tau217:
| PrecivityAD® | p-tau217 | |
|---|---|---|
| Ruling in patients also amyloid PET visual read positive (PPV) | 86% | 88% |
| Ruling out patients also amyloid PET visual read negative (NPV) | 78% | 92% ↑ |
| Overall percentage agreement with amyloid PET visual read | 81% | 90% ↑ |
- Both plasma-based biomarkers met the criteria for noninferiority to amyloid PET at a cutoff of 37 centiloids (CL) for selecting patients with amyloid pathology.
- “Results support the hypothesis that blood biomarkers may be interchangeable with amyloid-PET criteria for selecting patients who are suitable for and would benefit from treatment with novel amyloid-targeting therapies,” said Burnham.
Alzheimer’s disease blood tests of amyloid-beta 42/40, %p-tau217, 181, and 205 ratios and MTBR-243 in real-world populations: Results from SEABIRD and BioFINDER2 (Abstract ID: 88405)
Presenter: Randall Bateman (USA)
- Plasma microtubule binding region (MTBR)-tau243 shows good diagnostic accuracy for tau-PET positivity and a robust correlation with tau PET in the late Braak regions, which is consistent across patients with and without amyloid-β positivity.
| All participants n=108 | Amyloid-β-positive participants n=51 | |
|---|---|---|
| MTBR-tau243 | 1.00 | 0.98 |
| p-tau217/tau217 ratio | 0.98 | 0.87 |
| p-tau217 concentration | 0.98 | 0.89 |
| All participants n=108 | Amyloid-β-positive participants n=51 | |
|---|---|---|
| Braak I–VI (Global) | 0.87 | 0.86 |
| Braak I–II | 0.89 | 0.54 |
| Braak III–IV | 0.89 | 0.79 |
| Braak V–VI | 0.85 | 0.86 |
AUC: area under the receiver operating characteristic curve
- Correlation with Mini-Mental State Examination (MMSE) score found to be similar for MTBR-tau243 as for tau PET in all patients (Rho=–0.82 vs –0.69) and those with amyloid plaques (Rho=–0.54 vs –0.53).
Head-to-head evaluation of leading blood tests for amyloid pathology (Abstract ID: 95506)
Presenter: Kellen Petersen (USA)
Logistic regression models predicting the accuracy of blood-based biomarkers to predict amyloid-PET positivity (>20 CL) using AUC analysis and compared using DeLong’s tests.
| Platform | Model | AUC |
|---|---|---|
| C2N Precivity™ | p-tau217 ratio* plus amyloid-β 42/40 | 0.929 ★ |
| p-tau217 ratio | 0.927 | |
| p-tau217 plus amyloid-β 42/40 | 0.921 | |
| p-tau217 | 0.916 | |
| amyloid-β 42/40 | 0.751 | |
| Fujirebio Lumipulse® | p-tau217 plus amyloid-β 42/40 | 0.911 |
| ptau217 | 0.896 | |
| amyloid-β 42/40 | 0.787 | |
| AlzPath Simoa® | p-tau217 | 0.885 |
| Janssen Simoa® | p-tau217 | 0.882 |
| Roche Elecsys® | p-tau181 plus amyloid-β 42/40 plus GFAP plus NfL | 0.677 to 0.873 |
| p-tau181 plus amyloid-β 42/40 plus NfL | 0.677 to 0.873 | |
| p-tau181 plus amyloid-β 42/40 | 0.677 to 0.873 | |
| p-tau181 | 0.677 to 0.873 | |
| amyloid-β 42/40 | 0.677 to 0.873 | |
| GFAP | 0.677 to 0.873 | |
| NfL | 0.677 to 0.873 | |
| Quanterix Simoa® | p-tau181 plus amyloid-β 42/40 plus GFAP plus NfL | 0.670 to 0.808 |
| p-tau181 plus amyloid-β 42/40 plus NfL | 0.670 to 0.808 | |
| p-tau181 plus amyloid-β 42/40 | 0.670 to 0.808 | |
| p-tau181 | 0.670 to 0.808 | |
| amyloid-β 42/40 | 0.670 to 0.808 | |
| GFAP | 0.670 to 0.808 | |
| NfL | 0.670 to 0.808 |
AUC: area under the receiver operating characteristic curve; GFAP: glial fibrillary acidic protein; NfL: neurofilament light
*p-tau217 ratio of p-tau217 to non-p-tau217
A head-to-head comparison between plasma p-tau217 and tau-PET for predicting future cognitive decline among cognitively unimpaired individuals (Abstract ID: 90966)
Presenter: Rik Ossenkoppele (the Netherlands; Sweden)
Plasma p-tau217 predicts cognitive decline on the mini mental state examination (MMSE) and the modified Preclinical Alzheimer Cognitive Composite (mPACC).
Best predictive models:
- MMSE: plasma p-tau217 plus tau-PET uptake in the temporal neocortex (neoT; measured less than a year apart).
- mPACC: plasma p-tau217 plus tau-PET uptake in the medial temporal lobe (MTL; measured less than a year apart).
| Cognitive decline on MMSE | Cognitive decline on mPACC | |
|---|---|---|
| Plasma p-tau217 | R2 = 0.14 | R2 = 0.30 |
| MTL tau-PET | R2 = 0.17 | R2 = 0.32 |
| NeoT tau-PET | R2 = 0.21 | R2 = 0.31 |
Plasma Tau Biomarkers for Alzheimer’s Disease Staging (Abstract ID: 88408)
Presenter: Laia Montoliu-Gaya (Sweden)
- Five-step disease staging model proposed based on positivity for four plasma tau peptides (p-tau217, p-tau205, 0N CNS-specific, and tau 212–221) following quantification of six phosphorylated and six non- phosphorylated tau peptides in 553 plasma samples from participants in the BioFINDER-2 cohort.
- The model was compared with amyloid (A) and tau (T) PET status and PET stages in the early medial temporal lobe (MTL) and intermediate neotemporal (neoT) region.
| Plasma stage | A/T status | A- T- | A+ T- | A+ T+ | PET stage | MTL+ neoT– | MTL+ neoT+ | MTL+ neoT++ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma Stage | A/T Status | PET stage | ||||||||||||
| A- T- | A+ T- | A+ T- | MTL+ neoT- | MTL+ neoT+ | MTL+ neoT++ | |||||||||
| Stage 1: negative for all biomarkers | 80.8% | 19.2% | - | - | - | - | ||||||||
| Stage 2: p-tau217 positive | 3.4% | 40.9% | 55.7% | 22.6% | 22.6% | 31.0% | ||||||||
| Stage 3–4: p-tau217, p-tau205, and 0N CNS-specific positive | - | - | 100% | - | 83.3-86.7% | |||||||||
| Stage 5: p-tau 217, p-tau205, 0N CNS- specific, and tau 212–221 positive | - | - | 100% | - | - | 100% | ||||||||
- “Our data supports the notion of staging AD using fluid biomarkers,” said Montoliu-Gaya.
Predicting PET-Based Amyloid and Tau Pathology Stages With Plasma Biomarkers in Alzheimer’s disease (Abstract ID: 90581)
Presenter: Han-Kyeol Kim (Republic of South Korea)
The ratio of plasma p-tau217 to non-p-tau217 was superior to p-tau217 and the ratio of amyloid-β 42 to amyloid-β 40 for predicting Thal phase and Braak stage measured using PET, particularly in the early stages.
AUCs for plasma p-tau217/non-p-tau217 ratio were:
- 0.965 for predicting Thal phase I–II
- 0.848 for predicting Thal phase ≥III
- 0.864 for predicting Braak I–II
- 0.925 for predicting Braak III–IV
- 0.889 for predicting Braak V–VI
Mid- to late-life changes in blood-based biomarkers of Alzheimer’s disease pathology and neurodegeneration and associations with brain amyloid deposition: The ARIC-PET Study (Abstract ID: 91307)
Presenter: Priya Palta (USA)
Plasma biomarkers were measured in midlife, at a mean age of 58.5 years, or later life, at a mean age of 76.2 years.
Biomarkers tested:
- Ratio of amyloid-β 42 to amyloid-β 40
- Phosphorylated (p)-tau181
- Neurofilament light (NfL)
- Glial fibrillary acidic protein (GFAP)
Midlife plasma biomarkers: None of the biomarkers measured at mid-life predicted late-life PET amyloid positivity, defined as a global cortex standardized uptake value ratio of greater than 1.2, a median of 19.3 years later.
Later life plasma biomarkers: Significant associations with late-life PET amyloid positivity when measured a median of 1.6 years before for:
- Amyloid-β 40/42 ratio: odds ratio=2.12
- p-tau181: odds ratio=1.76
- GFAP: odds ratio=1.72
Anti-amyloid therapies
Key takeaways:
- Limited duration dosing recommended for donanemab beyond 12 months, which is best guided by visually negative PET scan rather than tau levels.
- Evidence, including biomarker data, supports early use of lecanemab and continued benefit through 3 years.
Insights from TRAILBLAZER-ALZ 2 (Donanemab): Potential Clinical Translation (Developing Topic Session)
Clinical efficiency
Presenter: Jennifer Zimmer (USA)
- At 76 weeks, donanemab had reduced the risk for disease progression to the next stage of Alzheimer’s disease (AD) on the Clinical Dementia Rating Global Score (CDR-GS; measured every 3 months) by a significant 37%.
- The percentage of patients progressing to moderate AD (CDR-GS score ≥2 points) at 76 weeks was reduced by a significant 50%.
- Efficacy was consistent across tau populations: the risk for disease progression among patients with low–medium tau and high tau was reduced by a significant 39% and 38%, respectively.
- Consistent efficacy across subgroups, including Hispanic/Latino participants.
Manageable ARIA risk
Presenter: Alessandro Biffi (USA)
- Amyloid-related imaging abnormalities-edema/effusions (ARIA-E) occurred in 24% of patients taking donanemab – 18% asymptomatic and 6% symptomatic.
- Serious ARIA-E events occurred in 1.5% of donanemab-treated patients, resulting in death in three (0.4%) patients.
- Highest ARIA-E frequency in apolipoprotein (APO)E ε4 homozygotes (40.6 vs 15.7% of noncarriers).
- Most patients only had one ARIA-E episode; 68% of patients were re-dosed with no recurrence in 70%.
- Recurrences of ARIA-E tended to be asymptomatic and mild to moderate.
- Main risk factors are:
- APOE ε4 genotype.
- Number of baseline microhemorrhages.
- Presence of cortical superficial siderosis at baseline.
- Risk managements recommendations:
- Identify higher risk patients prior to treatment.
- Adhere to MRI monitoring schedule.
- Titrate, interrupt, or discontinue treatment as required.
- Use corticosteroids for serious or symptomatic ARIA.
Limited duration dosing
Presenter: Emily Collins (USA)
- Amyloid levels remained low among patients who met dose completion criteria, with a re-accumulation rate of 2.8 centiloids (CL)/year.
- The mean time to switch to placebo was 47 weeks and the difference between treatment groups continued to widen beyond this point.
- Two-thirds of patients had amyloid PET levels below 24.1 CL by 12 months and this was consistent with a visually negative PET scan, which could be used to help determine when to stop treatment.
- Lower baseline amyloid levels predicted earlier reduction in amyloid PET to below 24.1 CL.
- Plasma p-tau was not accurate enough to determine donanemab amyloid removal because after amyloid removal it still reflects tau pathology.
Is there Evidence for a Continued Benefit for Long-Term Lecanemab Treatment? A Benefit/Risk Update from Long-Term Efficacy, Safety and Biomarker Data (Abstract ID: 92094)
Presenter: Christopher Van Dyck (USA)
Evidence of continued treatment effect with lecanemab through 3 years:
- At 36 months, the mean change in Clinical Dementia Rating-sum of boxes (CDR-SB) from baseline was 3.09 points, compared with a mean change of 1.20 points at 18 months.
- This translated to a significant 0.95-point difference in CDR-SB compared with matched historical controls from the ADNI study, an increase on the 0.45-point difference compared with placebo at 18 months, and a 30% reduction in the risk for progression to next stage of disease.
Low tau and amyloid groups do particularly well, supporting early lecanemab use:
- Among 58 patients with no or low tau PET (SUVR <1.06) at baseline, 59% had no decline in CDR-SB score at 36 months, and 51% had improvement.
- Among 151 patients with low amyloid PET at baseline (<60 CL), 46% had no decline in CDR-SB score and 33% had improvement.
Plasma biomarker data support continued lecanemab use:
- Improvement in amyloid PET and plasma amyloid-β 42/40 ratio within 3 months among patients starting lecanemab at 18 months.
- Continued treatment benefit from 18 to 36 months, despite approximately 70% of patients being amyloid negative (<30 CL) at 18 months.
- Plasma levels of p-tau217 decline in first 18 months with lecanemab treatment but increase with placebo.
- CSF MTBR-tau243 slowed by 44% after 18 months of treatment with lecanemab vs placebo.
Low long-term ARIA risk
- The exposure-adjusted rate of amyloid-related imaging abnormalities-edema/effusions (ARIA-E) was 6.8 per 100 person–years with lecanemab across the core study and extension phase, compared with 9.6 per 100 person–years for the core study alone.
- ARIA-E mainly occurs in the first 6 months of treatment, after which there are few cases and rates are similar to those with placebo.
- ARIA are not associated with accelerated long-term progression.
How Does the Latest Clinical Pharmacology Data & Modeling Support Continued Lecanemab Dosing? (Abstract ID: 92091)
Presenter: Larisa Reyderman (USA)
Study 201: 31 patients with early Alzheimer’s disease who stopped taking lecanemab (10 mg/kg every 2 weeks) after 18 months of treatment, before resuming a mean of 2 years later.
- The dual action of lecanemab means it not only clears plaques, but also targets highly toxic protofibrils that continue to form and cause Alzheimer’s disease pathology after amyloid is removed.
Interruption of lecanemab in Study 201 led to:
- 21% re-accumulation of amyloid PET.
- 47% worsening in amyloid-β 42/40 ratio.
- 30% re-accumulation of glial fibrillary acidic protein (GFAP).
- 24% re-accumulation of p-tau181.
- 13% re-accumulation of p-tau217.
Pharmacokinetic and pharmacodynamic modeling showed that half the treatment effect of lecanemab is lost:
- After 6 months for the amyloid-β 42/40 ratio.
- Within 12.1 years for amyloid PET.
- In 1.6 years for p-tau181.
- In 1.7 years for GFAP.
Monthly maintenance dose of lecanemab 10 mg/kg started at either 18 or 24 months is sufficient to prevent the re-accumulation of amyloid and worsening of plasma biomarkers.
- Had a comparable effect to bi-weekly dosing for 4 years in terms of clinical outcome measured by amyloid PET and CDR-SB.
Click below to download the Microdigest 2 pdf:
Download Microdigest 2 PDFYou can also download the Microdigest from AD/PD & CONy:
Download Microdigest 1 PDF





